CREATING CONFIDENCE
RESEARCH
Thanks to the continuous and ongoing research collaboration with many friends and colleagues in the world with renowened expertise we have been able to develop a unique model for establishing the viral transmission risk of NAT screened blood components.
It is the ambition of our company to provide a post-market performance follow up system of in vitro diagnostics (IVDs) for detection of blood borne pathogens in a way that the safety of blood products can be evaluated and controlled.
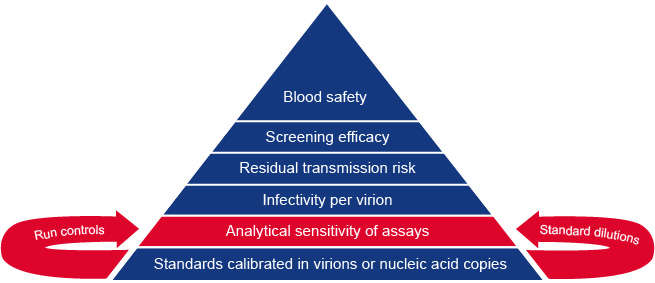
To achieve this goal it is necessary that the viral standards and controls for viral NAT are calibrated in nucleic acid copies or virion numbers. Thirty-five years of research in the area of blood safety has given us insight into how the analytical sensitivity of viral NAT (and serologic antigen) assays can be translated to lengths of diagnostic window periods and residual viral transmission risk by blood transfusion.
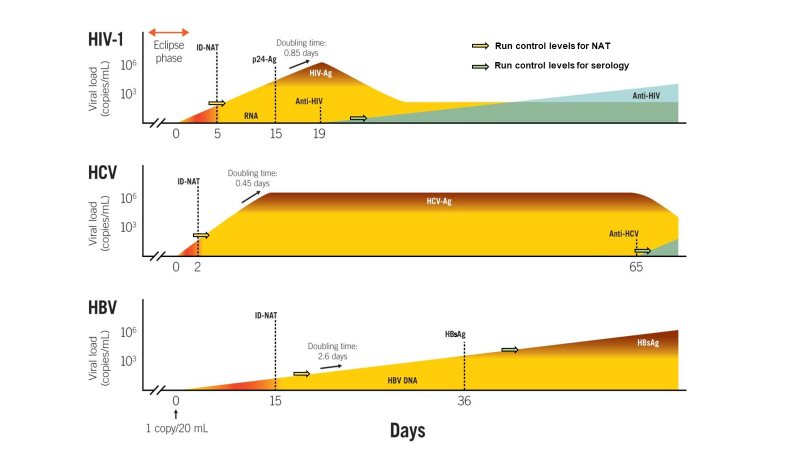
The foundations of our risk model for blood safety are explained in figure 1 and 2 below. Since our risk models make use of the viral load distribution and the 50% infectious dose in different stages of infection we can now estimate residual risk with alternative blood safety scenarios based on either viral serology, NAT and/or pathogen inactivation.
Learn more about the building blocks of our blood safety evaluation system listed on this page.